Production of Nitrogen-Containing Liquids From Hydrocarbon and N2 Feeds
Tech ID: 20-031
Inventors: Jason Hicks, Patrick Barboun, Deanna Poirier, Gerardo Rivera-Castro
Date Added: June 30, 2020
Overview
A plasma-assisted, catalytic one-step process that produces nitrogen containing compounds from natural gas
Technology Summary
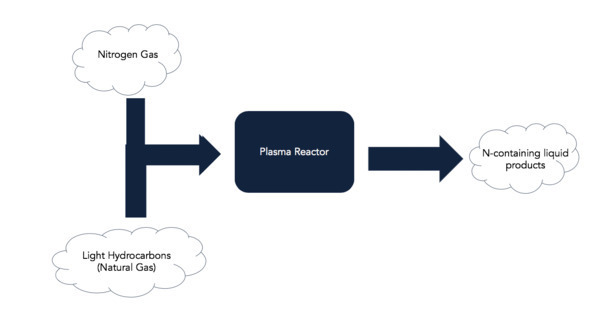
The United States relies on domestic natural gas production for a variety of residential, commercial, and industrial energy needs. According to the U.S. Energy Information Administration, the United States has abundant natural gas resources with about 464 trillion cubic feet (Tcf) of proved wet natural gases (which includes 308 Tcf of shale gas resources) and 438 Tcf of proved dry natural gas. The EIA also estimates the U.S. has 2,137 Tcf of unproved dry natural gas resources and that, especially as natural gas continues to supplant coal as a primary fuel for power generation, natural gas production will likely increase for decades. Natural gas is made primarily from hydrocarbons, a majority of which are used as fuels and some of which are converted into other chemicals. In particular, natural gas is an important feedstock for creating nitrogen-containing liquids from hydrocarbons and molecular nitrogen (N2) for drug manufacturing to the pharmaceutical industry. While there are multiple methods for making nitrogen-containing compounds from natural gas, all have their drawbacks, such as complicated synthesis pathways, low conversion efficiencies, and high energy requirements.
Researchers at the University of Notre Dame have developed a modular and flexible plasma-assisted catalytic process that converts natural gas to N-containing compounds in a single step process. Non-thermal plasmas create reactive environments, effectively loosening the thermodynamic equilibrium constraints that prevent hydrocarbon conversion into valuable products. Coupling careful control of the plasma properties and catalyst interactions allows for synthesis of N-containing heterocyclic aromatic hydrocarbons directly from shale gas at the wellhead for immediate transportation as liquids. This method has higher conversion efficiencies and is less energy intensive than current techniques.
Market Advantages
- Nitrogen containing hydrocarbons are more valuable than hydrocarbons
- Liquid product is better for capture and eases transportation issues
- Less energy consumption
- Cheaper production
Applications
Compounds important in synthesis of
- Natural compounds
- Medicinal chemistry
- Specialty chemicals/pharmaceuticals
- Green solvents such as ionic liquids
Technology Readiness Level
TRL 2 - Technology Concept Formulated
Intellectual Property
Patent Pending
Publications
Catalysis Enabled by Plasma Activation of Strong Chemical Bonds: A Review. Doi:10.1021/acsenergylett.9b00263
Contact
Richard Cox
rcox4@nd.edu
574.631.5158