Design, synthesis, & anti-TB activity of BTZ Azide & related click chemistry products
Tech ID: 14-059
Inventor: Dr. Marvin Miller, Dr. Rohit Tiwari
Date Added: June 10, 2021
Overview
A new group of reversible and noncovalent anti-TB agent inspired by BTZ043
Summary
Tuberculosis (TB) is one of the top 10 causes of death, killing about 2 million people worldwide annually. The emergence of multi drug resistant (MDR), extensive drug resistant (XDR) and, recently, totally drug resistant strains further emphasizes the desperate, growing need for new anti-TB agents. The discovery of 1,3-benzothiazin-4-ones (BTZs), especially BTZ043, led to the identification of several other classes of nitroaromatic compounds as anti-TB agents. However, the covalent structure and the presence of a nitroso group creates limitations on BTZ043.
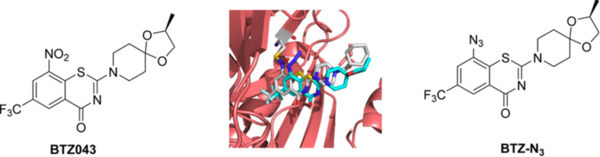
By substituting the nitro group of the BTZ043 scaffold by an electron-withdrawing azide group (N3), researchers at University of Notre Dame have developed BTZ-azide (Figure 1), a new group of anti-TB agents, with impressive inhibiting power against DprEi, an enzyme related to the cause of TB. While BTZ043 is a covalent inhibitor of DprE1, BTZ-azide is an efficient reversible and noncovalent inhibitor that provides further opportunities for development of much needed new anti-TB agents.
Market Opportunities
TB treatment market represents $916M (2016), growing at 5.2% annually
Technology Readiness Level
TRL 3- Experimental Proof of Concept
Intellectual Property Status
US 9,708,339: 1,3-benzothiazinone, sulfoxide, and sulfone compounds with electrophilic substituent
Publications
- Design, Syntheses, and Anti-TB Activity of 1,3-Benzothiazinone Azide and Click Chemistry Products Inspired by BTZ043
doi: 10.1021/acsmedchemlett.5b00424