Disease Driven Engineering of Multifunctional Nanoparticles
Tech ID: 12-018, 13-022
Inventors: Zihni Basar Bilgicer, Johnathan Ashley, Tanyel Kiziltepe-Bilgicer
Date Added: June 22, 2020
Overview
A novel method for constructing therapeutic liposomal nanoparticles enhances targeting and cellular uptake.
Technology Summary
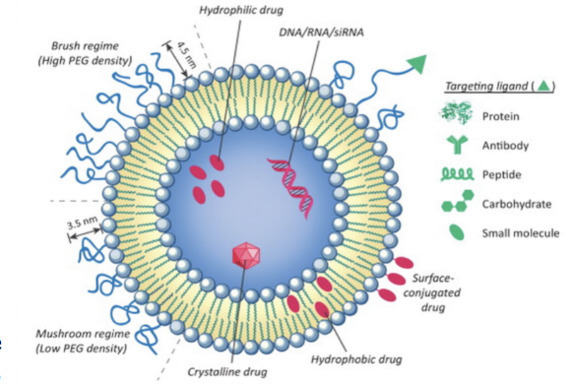
Chemotherapy is an effective and frequently utilized cancer treatment. Unfortunately, poor selectivity causes many chemotherapeutic drugs to attack both cancerous and noncancerous cells, resulting in toxic side effects and a decline in the patient’s quality of life. More recently, nanoparticles have been developed to provide more targeted delivery, but many lack versatility and fail the FDA approval process, further limiting feasible treatment options.
Researchers at the University of Notre Dame have developed a new class of effective liposomal nanoparticles, which are versatile structures that can integrate almost any drug and corresponding linkers to increase targeted delivery to cancerous cells. Composed of a lipid bilayer that forms in the shape of a hollow sphere, these dual-loaded liposomal nanoparticles (referred to internally as Lypos™ drug delivery nanoparticles) hold and shield the desired drug from the immune system, allowing it more time to reach the intended location. Unlike current nanoparticle production methods, which lack consistent drug loading and stability, this new drug delivery assembles the liposome lipid by lipid with specific drugs and targeting molecules that can be integrated at specified ratios, thus increasing the uniformity and selectivity, and decreasing off-target toxicity. Notre Dame’s new dual-loaded liposomal nanoparticles provide a reliable, timely, and cost-efficient way to increase the safety and efficacy of approved drugs and novel chemotherapies.
Market Advantages
- Increased selectivity of diseased cells which permits:
- Reduced dosages of cytotoxic drugs, and
- Reduced chemotherapeutic side effects
- Improved manufacturing consistency with specific drug and targeting molecule ratios
- Greater versatility with wide range of drug and targeting molecule accommodations
Technology Readiness Level
TRL 4 - Lab Validation
Intellectual Property Status
PCT/US2012/063614, PCT/US2014/014727 (Nano Particle Drug Delivery System)
PCT/US2016/051986 (Dual Loaded Liposomal Nanoparticles)
Publication
Rationally engineered nanoparticles target multiple myeloma cells, overcome cell-adhesion-mediated drug resistance, and show enhanced efficacy in vivo. doi:10.1038/bcj.2012.10.
Contact
Richard Cox
rcox4@nd.edu
574.631.5158